Softening Process In Water Treatment Plant . the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. It is these ions in hard water that. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. Softeners may also remove as. It is essential to remove contaminants in. softening through carbonate removal. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. the ion exchange water softening process can remove nearly all calcium and magnesium from source water.
from netsolwater.com
Softeners may also remove as. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. softening through carbonate removal. It is these ions in hard water that. It is essential to remove contaminants in. the ion exchange water softening process can remove nearly all calcium and magnesium from source water. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents.
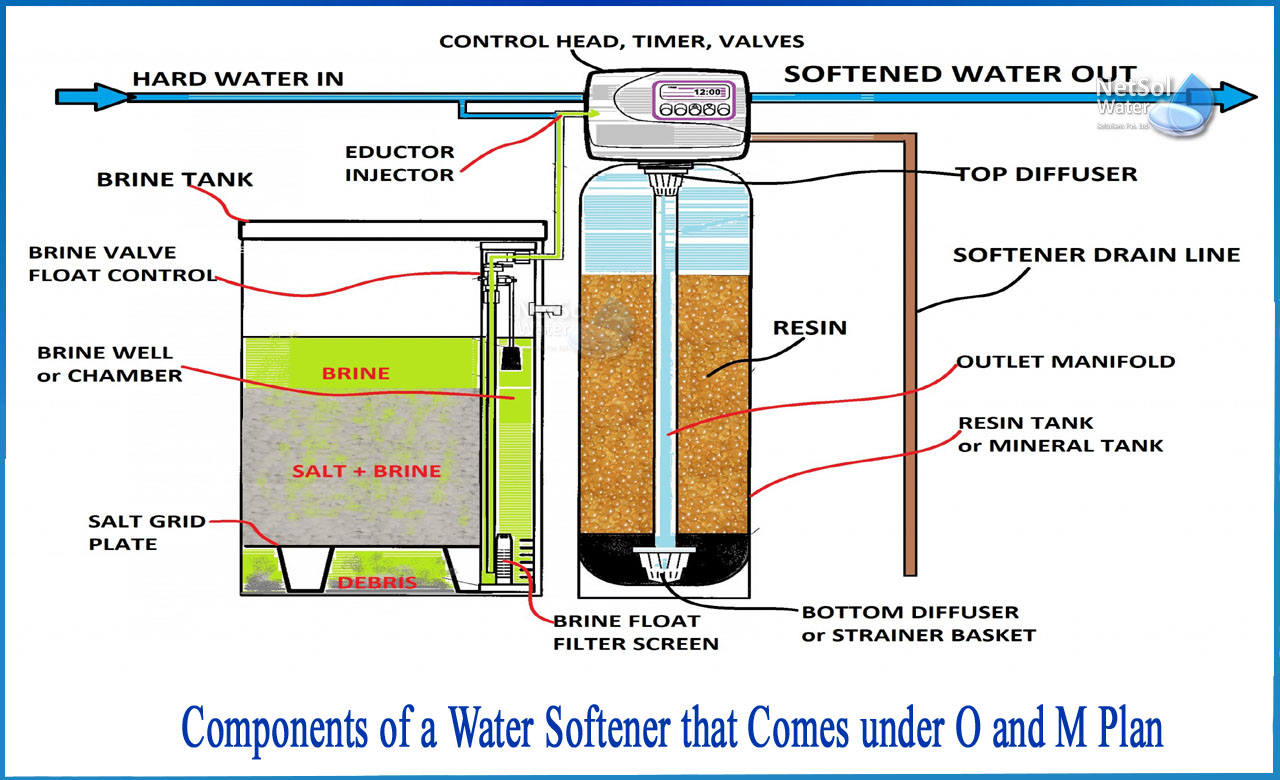
What are the components of a water softener under O and M Plan
Softening Process In Water Treatment Plant It is these ions in hard water that. It is these ions in hard water that. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. softening through carbonate removal. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. It is essential to remove contaminants in. the ion exchange water softening process can remove nearly all calcium and magnesium from source water. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Softeners may also remove as.
From watersoftenerfacts.ca
How Softeners Work Water Softener Facts Softening Process In Water Treatment Plant It is these ions in hard water that. Softeners may also remove as. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. the ion exchange water softening process can remove nearly all calcium and magnesium from. Softening Process In Water Treatment Plant.
From colemanhanna.com
Water Treatment, Reverse Osmosis & Water Softeners Coleman Hanna Softening Process In Water Treatment Plant the ion exchange water softening process can remove nearly all calcium and magnesium from source water. softening through carbonate removal. It is these ions in hard water that. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. Softeners may also remove as. water softening is a process in which the ions of. Softening Process In Water Treatment Plant.
From wteinfra.com
Industrial Water Softeners Plant manufacturer in India WTE Infra Softening Process In Water Treatment Plant water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. softening through carbonate removal. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. Softeners may also. Softening Process In Water Treatment Plant.
From netsolwater.com
What is water softening? Basic methods for water softening Softening Process In Water Treatment Plant It is these ions in hard water that. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. softening through carbonate removal. Softeners may also remove as. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. the four methods of ion exchange are. Softening Process In Water Treatment Plant.
From netsolwater.com
What are The Different Types of Water Softening Process Softening Process In Water Treatment Plant precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. It is essential to remove contaminants in. It is these ions in hard water that. softening through carbonate removal. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. water softening, the process of removing the dissolved calcium. Softening Process In Water Treatment Plant.
From netsolwater.com
What is demineralization process of water softening Softening Process In Water Treatment Plant It is essential to remove contaminants in. It is these ions in hard water that. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. water softening is a process in which the ions of calcium, magnesium. Softening Process In Water Treatment Plant.
From exobaxjnj.blob.core.windows.net
Lime Softening Water Treatment Plant at Daniel King blog Softening Process In Water Treatment Plant It is essential to remove contaminants in. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers,. Softening Process In Water Treatment Plant.
From upberi.com
How Does a Water Softener Work? + System Flow Diagram (2022) Softening Process In Water Treatment Plant the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. the ion exchange water softening process can remove nearly all calcium and magnesium from source water. water treatment is often required to make source water suitable. Softening Process In Water Treatment Plant.
From www.youtube.com
Water Softener Plant Operation I Softener water system I How does a Softening Process In Water Treatment Plant It is essential to remove contaminants in. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. softening through carbonate removal. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. It is these ions in hard water that. . Softening Process In Water Treatment Plant.
From www.korgentech.com
Water Softeners Water Softening Plants Softening Process In Water Treatment Plant water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. It is these ions in hard water that. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. precipitation. Softening Process In Water Treatment Plant.
From www.thewatertreatments.com
WATER TREATMENTS SOFTENER Water Treatment Waste Water Treatment Softening Process In Water Treatment Plant It is essential to remove contaminants in. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness. Softening Process In Water Treatment Plant.
From wiredatashineolawz.z4.web.core.windows.net
Ecopure Water Softener Installation Softening Process In Water Treatment Plant softening through carbonate removal. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. water softening is a process in which the ions of calcium, magnesium and sometimes iron. Softening Process In Water Treatment Plant.
From www.okwater.in
Water Softening Plant Ok Water System Softening Process In Water Treatment Plant It is these ions in hard water that. It is essential to remove contaminants in. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. Softeners may also remove as. softening through carbonate removal.. Softening Process In Water Treatment Plant.
From purewaterblog.com
The Water Softener Regeneration Cycle Explained! Water Treatment Softening Process In Water Treatment Plant water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. It is essential to remove contaminants in. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization.. Softening Process In Water Treatment Plant.
From www.envirogengroup.com
Water Softening Plant Envirogen Group Softening Process In Water Treatment Plant It is these ions in hard water that. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. softening through carbonate removal. the ion exchange water softening process can remove nearly all calcium and magnesium from source water. It is essential to remove contaminants in. precipitation softening processes are. Softening Process In Water Treatment Plant.
From www.elevate.in
Zeolitebased Monoliths For Water Softening By Ion, 59 OFF Softening Process In Water Treatment Plant the ion exchange water softening process can remove nearly all calcium and magnesium from source water. precipitation softening processes are used to reduce raw water hardness, alkalinity, silica, and other constituents. Softeners may also remove as. It is these ions in hard water that. softening through carbonate removal. water softening is a process in which the. Softening Process In Water Treatment Plant.
From wirewiringlorraine.z13.web.core.windows.net
Diagram Of A Water Treatment Plant Softening Process In Water Treatment Plant softening through carbonate removal. It is these ions in hard water that. water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. the four methods of ion exchange are water softening, deionization, demineralization, and dealkalization. It is essential to remove contaminants in. precipitation softening processes are used to reduce. Softening Process In Water Treatment Plant.
From apexenggroup.com
City of Fargo, NDLime SofteningWater Treatment Plant Apex Engineering Softening Process In Water Treatment Plant softening through carbonate removal. It is these ions in hard water that. water treatment is often required to make source water suitable for use in manufacturing processes, boilers, cooling towers, and rinse. Softeners may also remove as. water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. water softening. Softening Process In Water Treatment Plant.